Pomalid 4mg Capsules (Pomalidomide): Empowering Treatment for Multiple Myeloma
A novel drug, Pomalid 4mg Capsules (Pomalidomide), has proven to be highly effective in treating multiple myeloma, a difficult and aggressive form of cancer affecting plasma cells. Pomalid 4mg Capsules have become a potent therapeutic option for patients who have encountered disease relapse or refractory cases due to their distinct mode of action and demonstrated outcomes. With the development of this novel treatment, there is new hope for better outcomes and longer survival in the fight against multiple myeloma.
Understanding Multiple Myeloma:
A difficult form of cancer known as multiple myeloma is characterised by an abnormal increase in the number of plasma cells in the bone marrow. This condition interferes with the development of healthy blood cells, resulting in a number of symptoms including bone pain, exhaustion, anaemia, kidney issues, and an increased propensity to infections. Due to its complexity and resistance to conventional therapies, multiple myeloma treatment has long been a medical challenge.
The Role of Pomalid 4mg Capsules:
4mg of pomalid Pomalidomide, the active ingredient in the capsules, was developed especially to target and inhibit the growth of myeloma cells. It acts as an immunomodulatory medication, boosting the body’s defences against cancer cells while also upsetting the microenvironment that promotes their survival. In patients with relapsed or refractory multiple myeloma, pomalidomide has shown remarkable efficacy, providing a crucial treatment option when other therapies have failed.
Related Product
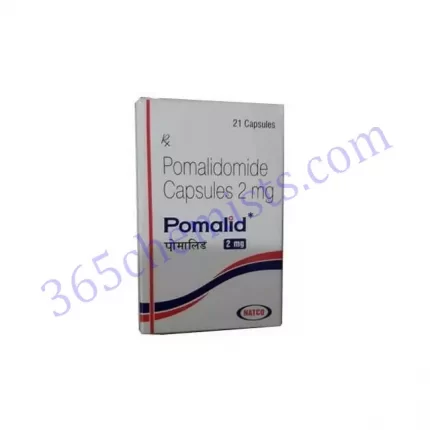
Pomalid 2mg Capsules
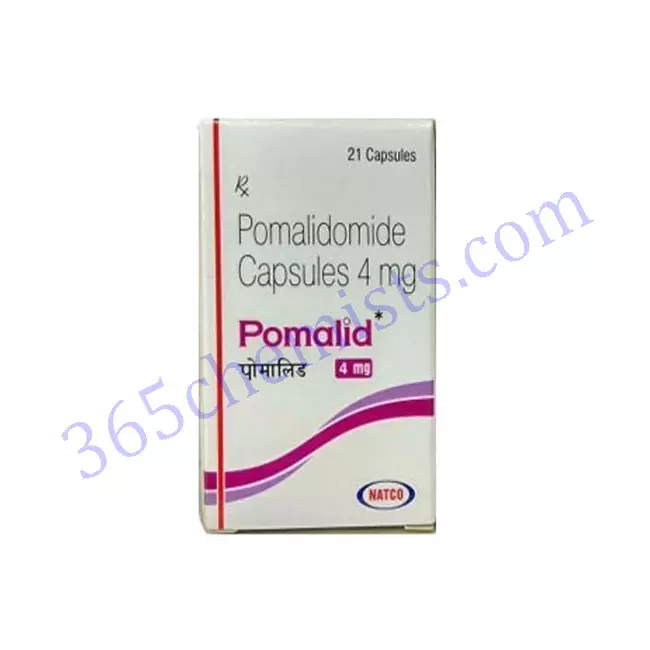
Pomalid 4mg Capsules
Mechanism of Action:
The therapeutic effects of pomalidomide are mediated by a complex mechanism. It slows down myeloma cell growth, interferes with how they interact with the bone marrow microenvironment, and improves the immune system’s capacity to identify and destroy cancer cells. Pomalid 4mg Capsules function as a potent tool against this relentless disease by attacking multiple pathways involved in myeloma cell survival.
Clinical Trials and Efficacy:
Pomalid 4mg Capsules have been shown to be safe and effective in treating patients with relapsed or refractory multiple myeloma in extensive clinical trials. In comparison to conventional therapies, these trials have demonstrated appreciable gains in progression-free survival, overall response rates, and quality of life. Pomalid 4mg Capsules have become more widely used in clinical settings thanks to regulatory approvals and encouraging study results.
Dosage and Administration:
Usually taken orally once daily, preferably on an empty stomach, Pomalid 4 mg capsules. Healthcare professionals will decide on the precise dosage and course of treatment based on the unique patient’s characteristics, such as disease stage, previous treatments, and general health. Patients should strictly adhere to the recommended regimen and speak with their healthcare team if they have any questions or concerns about their course of treatment.
Safety Profile and Side Effects:
While many patients have reported excellent tolerability with Pomalid 4mg Capsules, it’s important to be aware of any possible side effects. Fatigue, indigestion, constipation, nausea, anaemia, decreased appetite, and peripheral neuropathy are examples of frequent side effects. Patients must promptly inform their healthcare provider of any side effects so that appropriate management techniques can be used to reduce discomfort and maintain treatment efficacy.
Conclusion:
A significant improvement in the treatment of relapsed or resistant multiple myeloma is Pomalid 4mg Capsules (Pomalidomide). Patients dealing with this difficult disease have new reason for hope thanks to this medication’s focused mechanism of action, demonstrated efficacy, and manageable side effect profile. Pomalid 4mg Capsules is a testament to the unrelenting search for efficient treatments in the fight against multiple myeloma as ongoing research explores its potential applications and combination therapies.
Pomalid 4mg Capsules has developed into an essential weapon in the fight against multiple myeloma by preventing the growth of myeloma cells, disrupting the microenvironment that promotes their survival, and improving the immune system’s capacity to identify and destroy cancer cells.
With notable increases in progression-free survival, overall response rates, and quality of life for patients with relapsed or refractory multiple myeloma, Pomalid 4mg Capsules have demonstrated remarkable efficacy in clinical trials. Pomalid 4 mg Capsules have been approved and used more frequently in clinical settings as a result of these favourable results, giving patients who have tried all other forms of treatment new hope.
Pomalid 4mg Capsule dosage and administration are carefully chosen by medical professionals, taking into account specific patient factors. To maximise the efficacy of their treatment, patients must strictly adhere to the dosage recommended by their healthcare team.
Although Pomalid 4mg Capsules are typically tolerated well, it’s important to be aware of any possible side effects. The most frequent side effects include fatigue, indigestion, constipation, nausea, anaemia, decreased appetite, and peripheral neuropathy. Patients should notify their healthcare provider of any side effects as soon as possible so that they can offer direction and support in managing these symptoms.
The use of Pomalid 4mg Capsules (Pomalidomide) in the treatment of relapsed or resistant multiple myeloma represents a significant advancement. It is an excellent choice for patients in need thanks to its focused mechanism of action, demonstrated efficacy, and manageable side effect profile. Pomalid 4mg Capsules has the potential to improve outcomes and raise the quality of life for people with multiple myeloma with ongoing research and advancements.